
What is Calcium Chloride?
Calcium chloride is a direct application chemical widely handled, stored and used as an anti-freezing additive, deicing agent, and in dust control. Calcium chloride is an inorganic mineral compound made of elemental calcium Ca2+ and the halogen chlorine Cl–. These two elements combine to form a crystalline structure salt with the formula CaCl2. Additional names and identifiers for CaCl2 include calcium dichloride, calcium (II) chloride, liquid calcium and the CAS# 10043-52-4. Calcium chloride belongs to the halide family of common salt compounds and brine solutions along with sodium chloride NaCl and magnesium chloride MgCl.
Anhydrous Calcium Chloride

Anhydrous calcium chloride, meaning without water, is a white to white-gray solid often distributed as pellets, flakes, or as a granular powder. Dry CaCl2 has a high affinity and reactivity for water and can form various hydrate structures including mono-, di-, tetra-, and hexa-calcium chloride hydrates.
As a solid, CaCl2 is a strong desiccant effective in absorbing water and water vapor from its surrounding environment. Dry CaCl2 is highly soluble in water and the dissolving process is a measurable exothermic (i.e., heat-releasing) reaction. Calcium chloride’s chemical affinity for water and the heat produced on dissolution directly contribute to its ability to melt ice as well as reduce the freezing point of water.

Aqueous Calcium Chloride
When dissolved in water, calcium chloride is a colorless, odorless solution. Aqueous CaCl2 is also known as a metal-aquo complex due to the specific molecular interactivity between metal Ca2+ ions and water molecules. Liquid calcium is a stable, non-volatile solution often with a pH between 7-10.
Concentrated calcium chloride solutions are considered mildly corrosive and require proper handling and storage. Concentrations can range from dilute 1% up to saturated 42% by weight solutions. Commercial and industrially-produced products are common from 28% to 42% by weight. Liquid calcium at 29.8% strength is often used as it provides the lowest freezing point and greatest function as a deicing agent of the different calcium chloride solutions.
How Should Calcium Chloride Be Stored
Both solid and liquid calcium chloride are best stored in a dry, unventilated environment in an airtight, weatherproof container. Complete enclosure is recommended to limit air circulation across the stock that could potentially cause contamination or loss of strength through absorption of water vapor or carbon dioxide, CO2.
All tank components and associated equipment should be of materials with known compatibility. Calcium chloride is considered corrosive, incompatible and/or reactive to many metals including stainless steel, mild iron and galvanized steel.
Calcium Chloride Storage Preferences
Common material preferences for calcium chloride storage are within unreactive plastic, glass, and fiber reinforced plastics as these materials have known resistance to corrosion from CaCl2. PVC and other plastic piping as well as non-metallic chemical transfer pumps or diaphragm pumps are recommended for handling liquid calcium. Storage tanks should feature automatic pressure vents, manholes for maintenance access, and safety overflow pipes.
Calcium chloride is considered fairly stable for long term storage scenarios. Absorption of water vapor (dry CaCl2) or carbon dioxide (dry and liquid CaCl2) are the main concerns affecting long term storage. Solid and liquid forms are not sensitive to sunlight UV energy or high ambient temperatures. Bulk quantities are suitable for outdoor installation but hold a general preference for indoor storage.
Dry CaCl2
Stockpiles of solid calcium chloride are recommended to be kept indoors under waterproof cover to ensure chemical stability and avoid potential exposure to water such as from precipitation, excess humidity, and CO2.

Outdoor storage of bulk solids is recommended in fully sealed containers with suitable discharge engineering such as cone-bottom tanks to accommodate gravity feed of solid materials. For example, flakes of calcium chloride will require a 45° angle to achieve an effective outlet via gravity flow, and pellets will require a 35° angle to establish flow.
Liquid CaCl2
Liquid calcium is best stored in fully enclosed vertical chemical storage tanks. Certain concentrations are susceptible to low temperatures and freezing. Consider the following temperature ranges for CaCl2 solutions and the freezing points of low and high concentrations:

| CaCl2 Wt% | Freezing Point Range |
|---|---|
| 1% to 19% | 31°F to 6°F |
| 20% to 32% | -4°F to -17°F |
| 33% to 42% | 8°F to 69°F |
Liquid calcium solutions that are 20% to 32% by weight have subzero freezing points and are ideal for outdoor storage and applications that will experience very low ambient temperatures.
Solutions from 1% to 19% and from 33% to 42% are potentially sensitive to freezing, and bulk volumes are best kept indoors. If outdoor installation is intended for these concentrations, tank insulation and/or heat tracing may be needed.
The use of polyurethane insulation and/or heat maintenance can be used to prevent solution freezing during environmental cold exposure, and recommendation will depend on indoor or outdoor installation, geographic seasonal temps and the specific solution concentration required for the application.

Calcium Chloride Storage Tanks
Lined carbon steel, polyethylene, and fiber reinforced plastics are three storage tank materials commonly used in the bulk long-term handling of calcium chloride solids and liquids. The different tank types are compiled below based on an overview analysis of material durability, costs, service life and total compatibility. If considering polyethylene tanks, a quote can be requested for a calcium chloride tank that will meet any exact application design or insulation requirements.
Calcium Chloride Polyethylene Tanks

Both solid and aqueous calcium chloride stocks are well-suited for long term storage and handling in polyethylene tanks. Polyethylene (polyolefin) resins are naturally resistant to the corrosion concerns common with salts, brine storage, and environmental exposure. The resin forms of high density polyethylene (HDPE) and cross linked polyethylene (XLPE) are both considered inert to calcium chloride interactions and therefore ideal for long-term, repeat service applications.
Among polyethylene resins, the high-density HDPE variant is more frequently chosen for CaCl2 storage and is often used by winter maintenance crews for roads, airport runways, industrial facilities, corporations, and for large-scale dust control such as in mining and construction operations. In the U.S., many State Departments of Transportation report, recommend, and provide general guidelines for employees and contractors on handling calcium chloride and use in polyethylene storage tanks.
Poly Tank Advantages
Between material types, polyethylene has several advantages in addition to its chemical resistance. HDPE tanks provide a lower purchase cost, simple installation and placement, and ease of operation and integration into service equipment and worksites. Poly tanks provide a long service life and low replacement price, which makes them an effective asset with a highly valuable cost-to-service-life ratio. HDPE tanks are considered lightweight among chemical storage tanks and fairly easy to relocate as needed.
Poly tanks often feature top connective engineering that allows multiple tanks to be plumbed together to increase total storage capacity, especially when needing to provide system volumes in excess of 20,000 gallons. Calcium chloride polyethylene tanks must be manufactured according to federal regulations and design codes. Poly tanks intended for food grade applications must also meet FDA and U.S. CFR Title 21 requirements for polymers and additives used in extended food contact, indicating the products are of high quality.
Common CaCl2 Poly Tank Configuration
Common client and installation specifics for HDPE calcium chloride storage tanks include: containers are frequently 10,000 gallons in size or more, white in color, UV stabilized, fabricated to a 1.5 specific gravity rating or higher, and feature fittings, bulkheads, plumbing, and additional components made of PVC, EPDM, and/or Hastelloy materials. Calcium chloride tanks include vertical tanks, cone bottom tanks, and double wall tanks.
Fiber Reinforced Plastic Tanks

Calcium chloride is compatible with many options within the fiber-reinforced plastic category, known also as FRP composites. CaCl2 FRP tanks can be built from various resin materials such as vinyl, epoxy, polyethylene, or polypropylene. These resins form the base FRP polymer matrix that will be reinforced with fibers that are woven-in during fabrication.
Common conditioning fibers used for CaCl2 FRP tanks include glass and carbon. With composite materials, always verify the individual components’ compatibility with the intended cargo if extended contact is planned.
Overall, the FRP tank type is well suited for long term applications due to the stability, resistance, and longevity of the reinforced plastic material. When compared to other tank options, FRP tanks tend to be more costly and heavier than polyethylene but less than mild steel. FRP is not susceptible to environmental corrosion but is potentially more susceptible to impacts, drops and puncture damage. FRP tanks are typically custom made to size and often recommended when a larger storage volume in a single tank is needed.
Lined Carbon Steel Tanks

Unlined carbon steel is susceptible to corrosion from calcium chloride, making a protective liner required. Liquid calcium solutions in contact with mild steel will often discolor to a yellow, yellowish-brown due to iron ions being released as a result of chemical attack. This can be avoided, and successful storage can be achieved by installing an internal liner made from a chloride-resistant material.
Without a liner, corrosion can be expected in a steel CaCl2 tank between 2-20 mm/year with the rate increasing with more dilute solutions. With a compatible liner, carbon steel tanks become resistant to internal chloride corrosion. Common liner recommendations for CaCl2 include natural rubber, phenolics, vinyls, polyesters, and epoxy-based liners. Inspection of steel tanks and internal liners should be performed at regular intervals to ensure continuous vessel integrity during long term storage.
When compared to plastic and fiberglass containers, steel has an increased structural integrity against solution weight-strain, resistance to elevated temperatures, and durability against impacts and punctures. Carbon steel is perhaps a preferred material choice when needing volumes in large excess of 20,000 gallons. When compared to polyethylene storage methods, lined carbon steel tanks will have an increased cost of installation, can be susceptible to environmental corrosion, and are more difficult to relocate if ever necessary.
Calcium Chloride Storage Tank Components
Calcium chloride storage tank components, application equipment, fittings and plumbing should be resistant to the effects of ionic, chloride corrosion on repeated, continuous contact. Materials should be selected based on chemical compatibility test results that have evaluated their suitability for solid and liquid calcium chloride.
In general, plastics, rubber, and synthetic materials are often more resistant to ionic chloride solutions, such as CaCl2, MgCl2, NaCl, and HCl, over metal-based materials such as aluminum, cast iron, and steel.
The following material chart compiles chemical compatibility results for many common tank components and their suitability for calcium chloride applications.
| Material | Compatibility / Corrosion Effects |
|---|---|
| ABS Plastic | Good / Minor |
| CPVC | Excellent / None |
| PVC | Excellent / None |
| XLPE | Excellent / None |
| HDPE | Excellent / None |
| LDPE | Excellent / None |
| Polypropylene | Excellent / None |
| Nylon | Good / Minor |
| Neoprene | Fair / Moderate |
| PTFE (Teflon) | Excellent / None |
| PVDF (Kynar) | Excellent / None |
| Viton | Excellent / None |
| Hastelloy-C | Excellent / None |
| Fluorocarbon (FKM) | Excellent / None |
| Nitrile (Buna-N) | Fair / Moderate |
| EPDM | Excellent / None |
| Natural Rubber | Excellent / None |
| 304 SS | Good / Minor |
| 316 SS | Fair / Moderate |
| Aluminum | Not Recommended / Severe |
| Brass | Not Recommended / Severe |
| Carbon Steel | Fair / Moderate |
| Galvanized Steel | Not Recommended / Severe |
| Cast Iron | Fair / Moderate |
| Copper | Fair / Moderate |
| Titanium | Excellent / None |
Further CaCl2 Storage Considerations
The bulk storage of liquid calcium chloride and brine solutions may sometimes require secondary containment security measures. Secondary containment is an engineering fail-safe and control method put in place to contain the total volume of a tank or system of tanks should a release occur to protect personnel, the environment, and nearby facilities.
Acceptable containment methods for liquid CaCl2 include double wall tanks and spill dikes of impermeable, resistant materials, with concrete being a common example. Complete containment measures and storage limits are often regulated by local governments, and other specific requirements may be necessary, such as the proximity to surface waters. Always verify chemical storage systems are in adherence with local laws and guidelines.
Calcium Chloride Dissolution and Storage
Dissolving solid calcium chloride in water will produce heat, such as when needing to create a liquid solution for use. The amount of heat energy released is relative to total CaCl2 mass, form and hydration, and the amount of water volume. Using an example, heavy dissolutions involving flaked CaCl2 can raise water temperatures by as much as 80°F and pelletized CaCl2 as much as 150°F. In this example we are considering the on-site preparation of a liquid calcium solution in a bulk volume storage tank by dissolving solid CaCl2 in water.
Sudden temperature increases can be potentially hazardous to nearby workers and to the container system. Polyethylene materials can experience a weakening of tank structural integrity whenever holding fluids at temperatures above 120°F to 130°F. Poly tanks can be damaged and even fail resulting in catastrophic release from extended exposure to high temperatures and when they are without controls to limit or reduce temperature spikes.
Whenever dissolving calcium chloride, use cold water, monitor and account for associated temperature increases, know the temperature limits of the storage container, and consider dissolving in timed intervals to prevent boiling and potential container damage to ensure total storage integrity is maintained.
Calcium Chloride Properties
Calcium chloride is a nonvolatile chemical with a relatively high solubility in water. CaCl2 is not an active oxidizing agent, it is not flammable or combustible, and is not susceptible to degradation or UV breakdown on long term indoor or outdoor storage. Additional intrinsic chemical properties include calcium chloride’s hygroscopic and deliquescent nature, heat of dissolution, and ability to depress (i.e., reduce) the freezing point of aqueous solutions.
Calcium chloride’s properties of solubility, density, freezing and boiling temperatures vary on the particular CaCl2 solid form and hydration extent. For the liquid phase, these properties will vary on concentration strength.
The collective data for solid and liquid calcium chloride is compiled in the property tables below.
CaCl2 Solubility
Solid CaCl2 has a solubility of 745 g/L in water at 72°F and generally increases with the extent of hydration. To convert solubility, CaCl2 has a solubility of 196.8 grams/gallon or 0.434 pounds/gallon. Density for solids will range between 1.71 to 2.24 g/cm3, with a density of 2.16 g/cm3 for non-hydrated CaCl2. Non-hydrated CaCl2 has a melting point of 1424°F and boiling point of 3515°F.
Liquid Calcium Density and Specific Gravity
Liquid calcium measurements report density values of 8.32 lbs/gal to 11.97 lbs/gal and specific gravity values from 1.00 to 1.44 SG, with both values increasing with aqueous CaCl2 content. Liquid calcium chloride freezing and boiling points vary on weight percent strength.
Liquid Calcium Boiling and Freezing Point
Boiling points slowly increase from 100°F to 251°F at 42% by weight CaCl2. Calcium chloride freezing points are concentration specific, decreasing from 32°F to -67°F at 29.8%, the calcium chloride eutectic point, where the freezing point begins to rise to 69°F at 42%.
CaCl2 Affinity for Water
Calcium chloride is a hygroscopic solid. Hygroscopic chemicals have an affinity for absorbing and binding to available water, ice or vapor. Solid CaCl2 is also deliquescent, meaning it will draw enough moisture from its environment to eventually become a concentrated liquid brine. Calcium chloride is sensitive to atmospheric moisture and will begin to absorb water vapor whenever air is at 42% relative humidity (RH) and above.
These properties make solid CaCl2 effective as a desiccant and snow and ice melt, and dissolves more readily in water than other common salts due to being both hygroscopic and deliquescent. When compared to sodium chloride (NaCl, rock salt, commonly used for similar applications), NaCl does not absorb moisture until relative humidity is above 76% RH, dissolves into solution slower, and has a much lower heat of dissolution. This makes calcium chloride more effective than sodium chloride as a deicing agent.
CaCl2 Heat Release and Enthalpy of Dissolution
Calcium chloride’s increased affinity for water and ionic bond strength directly contributes to the amount of thermal energy (enthalpy, ΔH) released on dissolution. When solid, non-hydrated calcium chloride is dissolved in water, it separates into its elemental ions in an exothermic reaction that generates heat.
Measurements for this solution heat change (ΔH of dissolution, heat of solution) report around -82.8 kJ/mol of energy is released. This amount of energy is capable of raising solution temperatures and causing volume expansion. Caution should be exercised whenever adding CaCl2 to already-heated liquids as violent boiling can occur, which can lead to container overflow during tank filling or dissolution scenarios.
Freezing Point Depression
Aqueous calcium chloride has one of the lowest freezing points among commonly used brine solutions, as depicted in the property comparison graph. Calcium chloride ions in solution will decrease the freezing point of water depending on the total concentration. Freezing points reduce as calcium chloride strength increases, maximizing at 29.8% by weight.
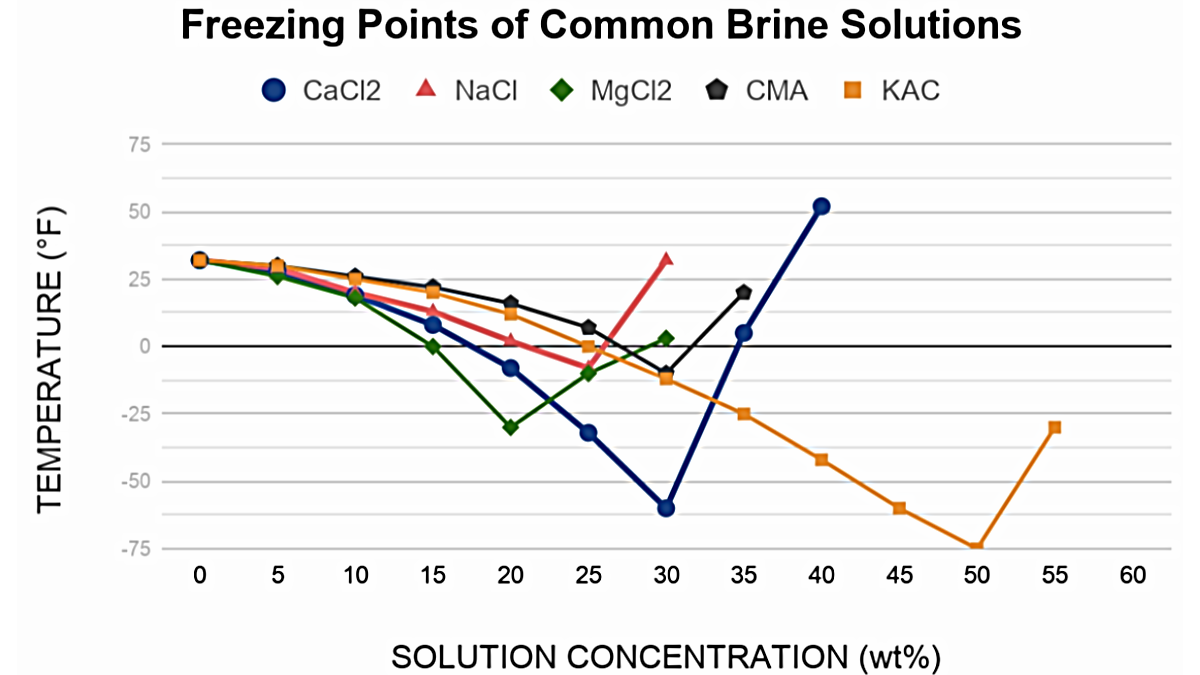
The ability of calcium chloride to depress the freezing point of water is most effective from 15 wt% and higher until the concentration reaches its chemical eutectic point of 29.8 wt%. At this strength, calcium chloride has its lowest freezing point of -67°F (-55°C). This makes such calcium chloride solutions highly effective in deicing, freeze-resistance as well as use in cold working mixtures and equipment that require or benefit from low temperatures.
Property Tables
Characteristic data for solid calcium chloride, its hydrate forms, and liquid concentrations have been compiled in the following property tables.
Solid Calcium Chloride and Hydrates
| CaCl2•nH2O: | Melting Point | Boiling Point | Density | Solubility |
|---|---|---|---|---|
| n = 0 [10043-52-4] | 1424°F (773°C) | 3515°F (1935°C) | 2.16 g/cm3 @ 77°F | 745 g/L @ 72°F |
| n = 1 [22691-02-7] | 369°F (187°C) | 361°F (183°C) | 2.24 g/cm3 @ 77°F | – |
| n = 2 [10035-04-8] | 349°F (176°C) | 345°F (174°C) | 1.85 g/cm3 @ 77°F | – |
| n = 4 [25094-02-4] | 114°F (45°C) | – | 1.83 g/cm3 @ 77°F | 908 g/L @ 72°F |
| n = 6 [7774-34-7] | 86°F (30°C) | – | 1.71 g/cm3 @ 77°F | 811 g/L @ 72°F |
| CaCl2•nH2O: | Heat of Fusion | Heat of Solution | Heat of Formation | Heat Capacity |
|---|---|---|---|---|
| n = 0 [10043-52-4] | 28.5 kJ/mol | -176.2 cal/g | -795.4 kJ/mol | 0.67 @ 77°F |
| n = 1 [22691-02-7] | 17.3 kJ/mol | -96.8 cal/g | -1109 kJ/mol | 0.84 @ 77°F |
| n = 2 [10035-04-8] | 12.9 kJ/mol | -72.8 cal/g | -1403 kJ/mol | 1.17 @ 77°F |
| n = 4 [25094-02-4] | 30.6 kJ/mol | -14.2 cal/g | -2010 kJ/mol | 1.35 @ 77°F |
| n = 6 [7774-34-7] | 43.4 kJ/mol | 17.2 cal/g | -2608 kJ/mol | 1.66 @ 77°F |
Liquid Calcium Chloride Solutions
| % CaCl2 | Freezing Point | Boiling Point | Specific Gravity | Density | Viscosity | Refractive Index |
|---|---|---|---|---|---|---|
| 0% | 32°F (0°C) | 212°F (100°C) | 1.00 | 8.32 | 1.02 cp | 1.333 |
| 1% | 31°F (-0.5°C) | 212°F (100°C) | 1.009 | 8.40 | – | – |
| 5% | 26°F (-3.3°C) | 213°F (101°C) | 1.045 | 8.70 | 1.07 cp | 1.345 |
| 10% | 20°F (-6.7°C) | 215°F (102°C) | 1.090 | 9.07 | 1.16 cp | 1.350 |
| 15% | 10°F (-12°C) | 217°F (103°C) | 1.139 | 9.48 | 1.40 cp | 1.372 |
| 20% | -4°F (-20°C) | 220°F (104°C) | 1.189 | 9.89 | 1.81 cp | 1.384 |
| 22% | -12°F (-24°C) | 222°F (106°C) | 1.209 | 10.06 | – | 1.392 |
| 24% | -20°F (-29°C) | 224°F (107°C) | 1.228 | 10.22 | – | 1.398 |
| 25% | -25°F (-32°C) | 225°F (107°C) | 1.240 | 10.32 | 2.38 cp | |
| 26% | -31°F (-35°C) | 226°F (108°C) | 1.251 | 10.41 | – | 1.401 |
| 28% | -46°F (-43°C) | 228°F (109°C) | 1.275 | 10.61 | – | 1.409 |
| 29.8% (eut.pt.) | -67°F (-55°C) | 230°F (110°C) | 1.294 | 10.77 | – | – |
| 30% | -52°F (-47°C) | 231°F (111°C) | 1.298 | 10.80 | 3.33 cp | 1.415 |
| 32% | -17°F (-27°C) | 233°F (112°C) | 1.322 | 11.00 | – | – |
| 34% | 10°F (-12°C) | 235°F (113°C) | 1.345 | 11.19 | – | – |
| 35% | 20°F (-6.7°C) | 238°F (114°C) | 1.357 | 11.29 | 4.99 cp | |
| 36% | 20°F (-6.7°C) | 239°F (115°C) | 1.369 | 11.39 | – | – |
| 38% | 48°F (8.9°C) | 240°F (116°C) | 1.392 | 11.58 | – | – |
| 40% | 61°F (16°C) | 247°F (120°C) | 1.416 | 11.78 | 8.48 cp | – |
| 42% | 69°F (21°C) | 251°F (122°C) | 1.439 | 11.97 | – | – |
Note: Specific Gravity, Density, and Viscosity Values are at 77°F. Density is reported in pounds/gallon (lbs/gal).
Incompatible Materials & Reactivity
Calcium chloride is a fairly unreactive and potentially long-term stable chemical. Solids are sensitive to available water with the potential for aggregation, clumping or eventual degradation to liquid brine. Both solids and solutions can be sensitive to atmospheric carbon dioxide (CO2), which can react with CaCl2 to form calcium carbonate and effectively reduce a stock’s total concentration and purity.
Calcium chloride solutions are considered incompatible with many metals including stainless steel. CaCl2 is known to increase the rate of metal corrosion due to the free chloride ions in solution and can cause pitting. The elemental metal atoms of many surfaces are sensitive to chloride reactions when water and oxygen are also present. This reactivity can result in a loss of material thickness, cause contamination of the solution and corrosion patterns specific to the metal and extent of incompatibility.
Galvanized steel and zinc coated products are known to be slowly attacked by calcium chloride solutions. This interaction will lead to the production of flammable hydrogen gas where sufficient accumulation can be potentially combustible, therefore posing a significant hazard in closed tank systems.
Additional specific CaCl2 incompatibilities include: bromine trifluoride, boric acid and calcium oxide are known incompatibles; methyl vinyl ether on exposure can undergo hazardous self-polymerization; CaCl2 solids can release toxic vapors of chlorine on elevated-heat decomposition.
Calcium Chloride Production
Calcium chloride is manufactured worldwide in both its solid and aqueous form for various uses across modern applications. Mass production annually exceeds at least 1.5 million tons in North America, 300,000 tons in Europe and 250,000 tons in Asia. There are three main methods of calcium chloride production: refining natural brine sources, the Solvay Process, and chemical substitution reactions that often involve limestone acid neutralization. Common solution impurities include residual lime, magnesium, and/or other alkali salts such as sodium chloride.
- Natural Brine Refining – Geological brine formations can be developed and mined for the elemental resources they contain. Many salt-mineral compounds, such as calcium chloride, are refined from natural brine formations found in groundwater, deep sediment basins, as well as seawater and saline lakes. Brine refining is the most common CaCl2 production method, especially in the USA where the Solvay Process is no longer used.
- The Solvay Process – Also known as the ammonia soda process, this method uses sodium chloride (NaCl, rock salt) and limestone (CaCO3, calcium carbonate) to produce sodium carbonate (NaCO3, soda ash) and calcium chloride. CaCl2 is made as a byproduct of this reaction that can then be separated and concentrated for distribution and use.
- Limestone Acid Neutralization – Natural limestone can be reacted and neutralized to yield aqueous calcium chloride and carbon dioxide as a gas byproduct. This process often uses hydrochloric acid (HCl) as the main reactant and is perhaps the least common for producing CaCl2.
Calcium Chloride Applications
Calcium chloride is used in many applications across modern society’s infrastructure, equipment, commerce, and industry in both its solid and aqueous forms. Calcium chloride has been used in North America as a freeze protection and dust control agent since the 1940s.
The majority of reported calcium chloride use is administered across roads and walkways for deicing and winter freeze protection. Dust control and road stabilization is the second most common application, followed by industrial processing, petrochemical oil wells use, and construction uses.
| CaCl2 Use | Percent |
|---|---|
| Deicing | 22% |
| Road Stabilization | 20% |
| Industrial Processing | 20% |
| Oil & Gas Wells | 17% |
| Concrete Additive | 12% |
| Miscellaneous | 9% |
- Freeze Protection – Calcium chloride is used as a solid, liquid and/or mixed with other salts as a deicer to melt ice and to prevent ice formation. CaCl2 is often layered across highways, roads and walkways to prevent ice accumulation, melt snow, and provide traction control for transportation and mobile safety.

Several common salt-brines are effective in freeze protection with some scenarios preferring calcium chloride due to its specific chemical properties.
(For government agencies, municipalities, and industry professionals, more info on calcium chloride and other brines frequently used in wintering applications can be found in this manual from the Federal Highway Administration of the US DOT.)
- Dust Control – Solutions of liquid calcium are sprayed over select roadways and infrastructure (dirt, gravel, shoulders and embankments) to limit the generation of airborne dust particles, often according to local regulatory oversight. The use of CaCl2 in dust control is common in quarries and other surface mining applications that feature large, heavy machinery.

- Industry Processing – Calcium chloride is used in various manufacturing and industrial production scenarios in both its solid and liquid forms. CaCl2 is useful in processing as a coagulant, a drying agent, a refrigerant in heating and cooling systems, or as a chloridizing agent in metallurgy, as a few examples.
- Oil & Gas Wells – Petrochemical and natural gas applications may use liquid calcium chloride as an oil recovery aid or as an additive in the injection fluids often associated with the drilling and complete installation of oil wells.

- Miscellaneous – Additional applications for calcium chloride include: as an additive in concrete to accelerate drying; in tires as a weight ballast and freeze-protectant; as a desiccant; in agriculture as an herbicide; as a food or beverage additive, anti-caking and firming agent; in the treating of fruits and vegetables for preservation; in medications and laboratory reactions, and as an additive and processing aid in plastics manufacturing.



Calcium Chloride Safety & Handling
Aqueous calcium chloride solutions can be slightly alkaline with a pH range of 7.0 to 10.0 depending on concentration and potential additives or impurities. The corrosive activities of CaCl2 can increase if solution pH falls beneath 7 – 6.5. Alkaline buffers can be added to stabilize and raise pH in such cases. Optimal pH is in the 7.5 – 9.5 range to reduce corrosion concerns for mild steel tanks, pipes, and/or fittings.
Calcium chloride is not a hazardous material as defined per UN / DOT shipping and handling regulations. However, solids and solutions of CaCl2 can be harmful on direct physical contact, which should be avoided, and protective equipment worn. Solid calcium chloride can be corrosive, and its hygroscopic nature can present extra hazards to sensitive tissues such as the eyes. Handling hazards associated with liquid CaCl2 will depend on its solution strength, increasing up to its maximum concentration in water.
Aqueous and solid calcium chloride is capable of moderate to severe eye and/or skin irritation or damage, especially with extended contact. Proper handling and protective equipment will be selective to the specific CaCl2 concentration, type and total volume, and should always be done according to accompanying product material safety data sheets and SOPs for bulk transfer or handling operations.
Takeaway | Calcium Chloride Storage and Handling 101
Calcium chloride is a hygroscopic and deliquescent chemical highly effective in freeze-point reduction, ice-melting applications, and as a desiccant and drying agent. Collectively, calcium chloride is a versatile, fairly safe and stable ionic molecular compound and chemical.
For storage and handling, polyethylene, PVC, EPDM and Hastelloy are common material choices for calcium chloride storage containers and components. Metal materials are known to be sensitive to CaCl2 corrosion. Dry CaCl2 can be prone to moisture contamination, and certain liquid strengths can be prone to freezing, making consideration of individual scenarios important in the compatible and long-term storage of calcium chloride.
When planning to purchase and implement a calcium chloride storage tank or tanks, communicate with equipment providers specific conditions such as location, ambient temperature ranges, total volumes and CaCl2 concentration as they can influence the recommendations for successful storage and equipment.
If you need assistance or would like more information on calcium chloride storage tanks or secondary containment options, contact our support experts. Our knowledgeable team of tank professionals work directly with the top providers, clients, and consumers in North America and will work directly with you to provide the chemical storage solution you need.

Calcium Chloride Storage Tanks

Professional Support & Product Selection
External Sources
- Calcium Chloride | CaCl2 | CID 5284359 – PubChem (nih.gov)
- Calcium Chloride | A Guide to Physical Properties – OxyChem (oxy.com)
- Metal Aquo Complex – Wikipedia (wikipedia.org)
- Recommended Anti-Icing Practices | Manual of Practice for An Effective Anti-Icing Program, June 1996 – FHWA (dot.gov)
- Salt and Brine Storage Guidance (michigan.gov)
- eCFR :: 21 CFR Part 177 | Indirect Food Additives: Polymers (ecfr.gov)
- Fibre Reinforced Plastic – An Overview | ScienceDirect Topics (sciencedirect.com)
- Calcium Chloride | A Guide to Handling and Storage – OxyChem (oxy.com)
- Secondary Containment Guidance – Michigan Department of Environment, Great Lakes, and Energy (michigan.gov)
- Calcium Chloride Handbook – DOW (studylib.net)
- Deliquescence | Water Absorption, Hygroscopy, Solutions – Encyclopedia Britannica (britannica.com)
- Comprehensive Evaluation of Bridge Anti-Icing Technologies | Final Report – Alaska DOT (alaska.gov)
- Enthalpy of Solution – Chemistry LibreTexts (libretexts.org)
- Calcium Chloride – CAMEO Chemicals (noaa.gov)
- Manual of Practice for an Effective Anti-Icing Program -FHWA (dot.gov)
- Carbon Dioxide Sequestration to form Calcium Carbonate Nanoparticles – Purdue University (purdue.edu)
- Handbook of Corrosion Data – ASM International
- CFR 2010 Title 21 Vol 3 Section 184-1193 (govinfo.gov)
- Production and Value of Minerals and Mineral Products in Michigan – State of Michigan (michigan.gov)
- Calcium Chloride NOSB Materials Database (ams.usda.gov)
- Solvay Process – Wikipedia (wikipedia.org)
- Acid Neutralization with Lime (lime.org)
- Recommended Anti-Icing Practices – Appendix A: Selected Chemicals and Their Properties – FHWA (dot.gov)
- Markets and Uses for Calcium Chloride – Peters Chemical Company (peterschemical.com)
- Prevention of Calcium Carbonate Precipitation from Calcium Chloride Kill Fluid in CO2-Laden Formations – SPE Western Regional Meeting | OnePetro (onepetro.org)
- Calcium Chloride | Crops (ams.usda.gov)
- Effects of the Use of Different Temperature and Calcium Chloride Treatments during Storage on the Quality of Fresh-Cut “Xuebai” Cauliflowers – PMC (nih.gov)
- Code of Federal Regulations | Hazardous Materials Table (govinfo.gov)
- Calcium Chloride | CaCl2 | Safety and Hazards – PubChem (nih.gov)


